During the development of all animals, tissues must grow in a coordinated manner in order to function properly as integrated units within the adult. This is especially important during neuronal wiring. In the case of motoneurons, axons must project over large distances, find a specific target muscle and then maintain appropriate synaptic efficacy during subsequent growth. The latter issue is particularly important during Drosophila neuromuscular development since the muscle volume increases up to 100 fold during larval growth. An inability to coordinate synapse growth with this rapid increase in muscle volume would likely lead to severe impairment of motor control. In the last few years, we have found that BMP signaling also regulates the growth and function of the synapse at the Drosophila neuromuscular junction (NMJ). This is a striking demonstration of the principle that signaling pathways are adapted during evolution for new purposes.
Our discovery that BMPs are involved in synapse growth came from studying the phenotype of mutations in the wishful thinking (wit) gene, a Drosophila homolog of the vertebrate BMP type II receptor. Mutations in wit prevent adult animals from hatching out of the pupal case. We showed that this phenotype is caused by reduced growth of motoneuron synapses at the NMJ and severe defects in neurotransmission. While these results revealed a novel role for BMP signaling in regulating Drosophila NMJ synapse growth and activity, they did not address the source of the signal that stimulates this pathway. In collaboration with the Goodman group at UC Berkley we showed that Gbb, a BMP 5/6/7 homolog, signals in a retrograde manner (from the muscle to the neuron) to regulate synaptic size and function at the Drosophila NMJ. Like mutations in wit, larvae mutant for gbb exhibit small synapses, reduced neurotransmission and defects in the ultrastructure of synaptic active zones. However, unlike Wit, which is specifically required in the presynaptic (neuronal) cell, we showed that restoring gbb expression in muscles, partially rescues the gbb synaptic defects. These results reveal that target -derived BMP signals are required to properly coordinate muscle and synapse growth at the Drosophila NMJ.
Since synapse growth has been implicated as one means by which learning is accomplished in higher organisms, we have decided to explore whether BMP signaling regulates synaptic plasticity in the vertebrate brain. To this end, we have been making hippocampus specific knockouts of various BMP signaling components in the mouse brain. The hippocampus regulates certain aspects of learning and memory in both the human and rodent brain. Our preliminary results using chordin knockout mice (Chordin is the vertebrate homolog of the BMP inhibitor Sog) suggest that BMP signaling does indeed regulate aspects of cellular memory. These studies are now being expanded to include analysis of BMPRII receptor mutants. BMPRII is the vertebrate counterpart of Wit, and we have found that it is heavily expressed in the hippocampus of adult mice. We expect that these studies will further our understanding of the signaling pathways that influence the process of learning and memory.
Click on the image below for a larger version
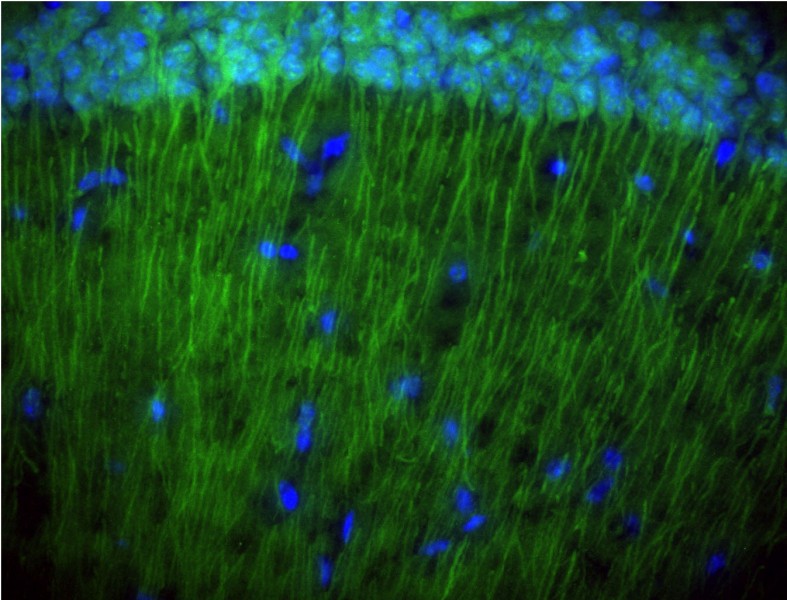
A mouse brain slice showing expression of Psmad (in green) in the dendrites of hippocampus nerons.
The neron cell nuclei are in blue (M. Sun)