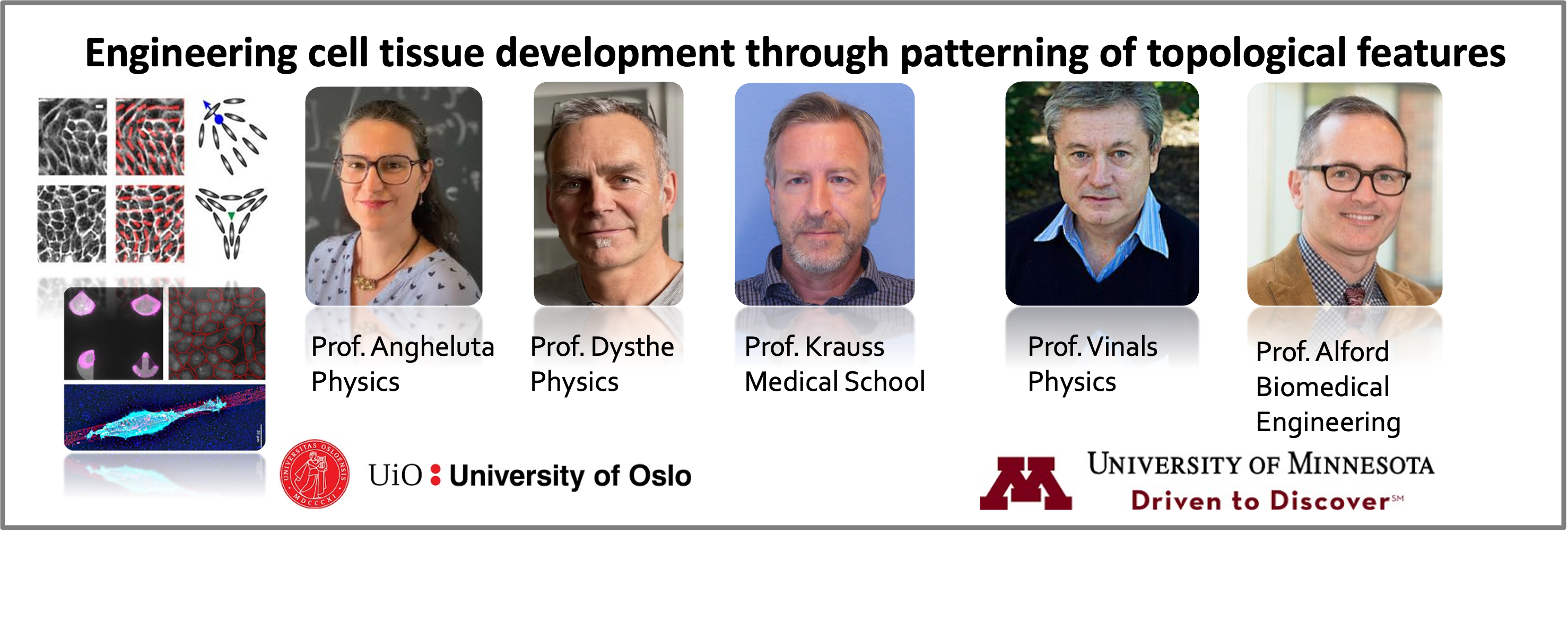
Current transatlantic research teams (2023-2025)
In fall 2023, four new teams were selected to initiate research projects.



Past research teams - 2020-2022
Transatlantic research teams (2020-2022)
In May 2020, six new teams were selected to initiate research projects (click here for project abstracts).





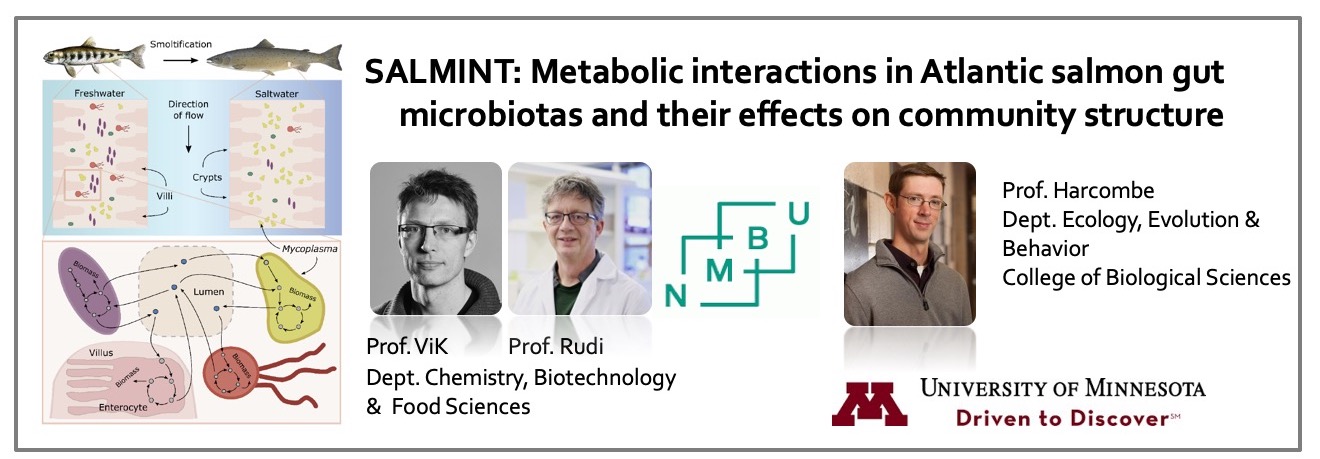
Past research teams - 2018-20
In May 2018, seven new teams were selected to initiate research projects (click here for project abstracts) starting in the Summer of 2018.






Past research teams
Past research teams - 2016-18
In June 2016, six teams were selected to initiate new research projects (click here for project abstracts) starting in the Fall of 2016.





Past research teams - 2015-17
In June 2015, a first group of transatlantic research teams were selected. A listing of the researchers and their projects is shown below.
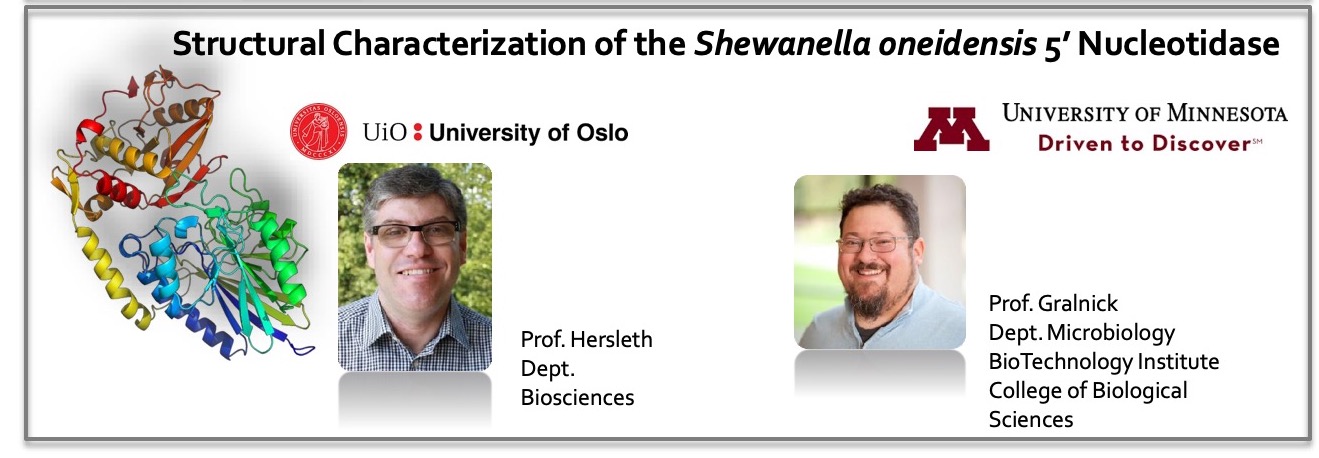
Structural characterization of the manganese transporter MntH of Escherichia coli
Robert Brooker, University of Minnesota | Preben Morth, University of Oslo
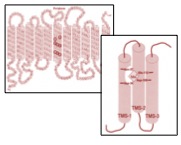
Iron and manganese function as essential cofactors for a variety of enzymes and protein complexes found in all living organisms. Among a wide range of species including humans, a major pathway for iron and manganese uptake is via membrane proteins called the Nramp family. Nramp2 in humans functions as the major pathway for the dietary uptake of iron into intestinal cells. This transporter is involved in diseases affecting iron metabolism, such as hereditary hemochromatosis and anemia. Nramp proteins in pathogenic bacteria also play an important role in their ability to cause disease. Our research is aimed at understanding the structural basis for iron and manganese uptake into cells. The goal of the proposed research is to determine the structure of MntH using X-ray crystallography. MntH and other Nramp proteins share no significant sequence identity with any of the membrane transporters that already have been determined by X-ray crystallography.
Thermodynamics and kinetics of bacteriocin mechanism of action
Yiannis Kaznessis, University of Minnesota | Dzung Diep, Norwegian University of Life Sciences | Jon Nissen-Meyer, University of Oslo

Antibiotics have changed the course of human history, saving innumerable lives and substantially improving the quality of life across the globe. Bacteria are fighting back though. Superbugs, species that do not respond to our most potent drugs, are evolving, infecting humans with alarming frequency. And our drug war chest is not being updated with new antibiotics because of a financial disincentive: one dose, one treatment with antibiotics may cure the disease, in stark contrast to medicines prescribed for life. We will borrow from nature and harness the potential of bacteriocins, naturally occurring antimicrobial peptides (AMPs).These are small proteins that lyse and kill bacteria rapidly. Experimental evidence strongly suggests that bacteriocins act primarily through specific interactions with membrane receptors and subsequent disruption of the cell membranes of target pathogens. We will investigate in detail how bacteriocins interact with bacterial membranes, determine the structural elements that make the peptides active against certain pathogens, and engineer new peptides that may have therapeutic potential.
Establishing a novel mouse model to study APOBEC-catalyzed DNA damage and its misrepair as a major source of mutation in cancer
Hilde Nilsen, University of Oslo | Reuben Harris, University of Minnesota

Many mutations must occur for a normal cell to become cancerous and for primary tumors to become aggressive and life threatening. Apart from obvious environmental and dietary sources, very little is known about the molecular origins of most cancer-causing mutations. The Harris laboratory at the University of Minnesota recently identified a DNA mutating enzyme called APOBEC that provides mutational fuel in the majority of breast cancers. The Nilsen laboratory at the University of Oslo is expert in the DNA repair enzymes that normally work to counteract such mutational activity. Here, we are working together to develop a new animal model for cancer research by determining whether mice with APOBEC and defective DNA repair enzymes show an elevated incidence of cancer. If so, such animals may be instrumental for future preclinical studies of new cancer drugs.
The role of phosphoinositide binding proteins in autophagy and disease
Thomas Neufeld, University of Minnesota | Anne Simonsen, University of Oslo
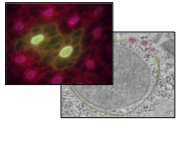
Eukaryotic cells possess the ability to engulf and degrade portions of their own cytoplasm through a process called autophagy. By promoting the turnover and recycling of cellular components, autophagy has been found to have a major impact on a range of human health and disease states including obesity, cancer, aging, neurodegenerative diseases, and inflammatory disorders. The ability to exploit autophagy as a novel therapy for a variety of diseases is an exciting prospect, but requires an improved understanding of how autophagy is regulated in the cell. This research project uses cultured human cells and in vivo studies in the fruit fly Drosophila to investigate a family of signaling molecules that regulate membrane remodeling and trafficking during autophagy.
Supply chain analysis of United States and Norwegian bioenergy policies
Dennis Becker, University of Minnesota | Erik Trømborg, Birger Sølberg, Hanne Sjølie, Berit Lindstad, Norwegian University of Life Sciences

The United States is in the midst of a rapid proliferation of policies to create a bioeconomy that simultaneously addresses national security concerns, forest health, and economic development. Norway is equally eager to increase development but for reasons linked to carbon mitigation and energy diversification. In both countries, the level of production falls well short of national goals despite significant investments. The goal of this project is to stimulate policy innovation and financial investments in forestry bioenergy production by building understanding of the mix of policy instruments commonly used at each supply chain step (e.g. tax incentives, regulations); the extent to which US and Norwegian policy structures result in different levels of bioenergy production; and which components of those policy structures can be effectively translated to one country or the other.
Using Lidar to enhance forest inventories
Ronald McRoberts, University of Minnesota | Erik Næsset, Terje Gobakken, Norwegian University of Life Sciences
Lidar (light detection and ranging) is an optical remote sensing technology that can be used to estimate the vertical distribution of tree volume for a ground location. A large, unique reference dataset will be constructed and used to support three projects on using lidar and inventory plot data to enhance forest inventories. A lidar-based map of growing stock volume for St. Louis County, Minnesota, will be used to construct stratifications for increasing the precision of inventory estimates. Our recent Norwegian study suggests that precision can be increased by a factor of 4-5, an increase that would otherwise cost more than $2.5 million in increased sample sizes. Optimization of lidar techniques will be investigated for carbon accounting and for estimating forest change. A successful outcome means that lidar enhanced inventories may be operationally implemented almost immediately.