
“At the U of M, you’ve got the opportunity to ask a question, and then answer it in the best way you can, rather than building your research around where you're pigeon-holed.”
If you were a kid on a quest to build an awesome Lego stack, you’d start with a pile of disorganized bricks. Carefully, you’d grab bricks one by one and snap them into a linear structure, until your stack was tall enough. Naomi Courtemanche, new faculty hired on in the Cellular Biophysics cluster, studies how a similar type of construction happens within living cells—but instead of Legos and kids, she investigates the dynamic relationship between actin and formin molecules.
Actin monomers are proteins that can polymerize into linear strands called filaments. Individual filaments are often bundled into strong cables that play critical roles in cytoskeletal interactions, cell division, and cell motility. Actin, however, does not easily polymerize on its own. A different molecule, formin, helps to assemble actin—just as a child has to construct a stack of Legos.
With her background in physics and math, Courtemanche is drawn to research approaches that strive to understand nature by using physical principles. “There is a core polymerization principle here, which is a kinetic and thermodynamic process,” says Courtemanche of the actin-formin connection. “You can understand it using equations.”
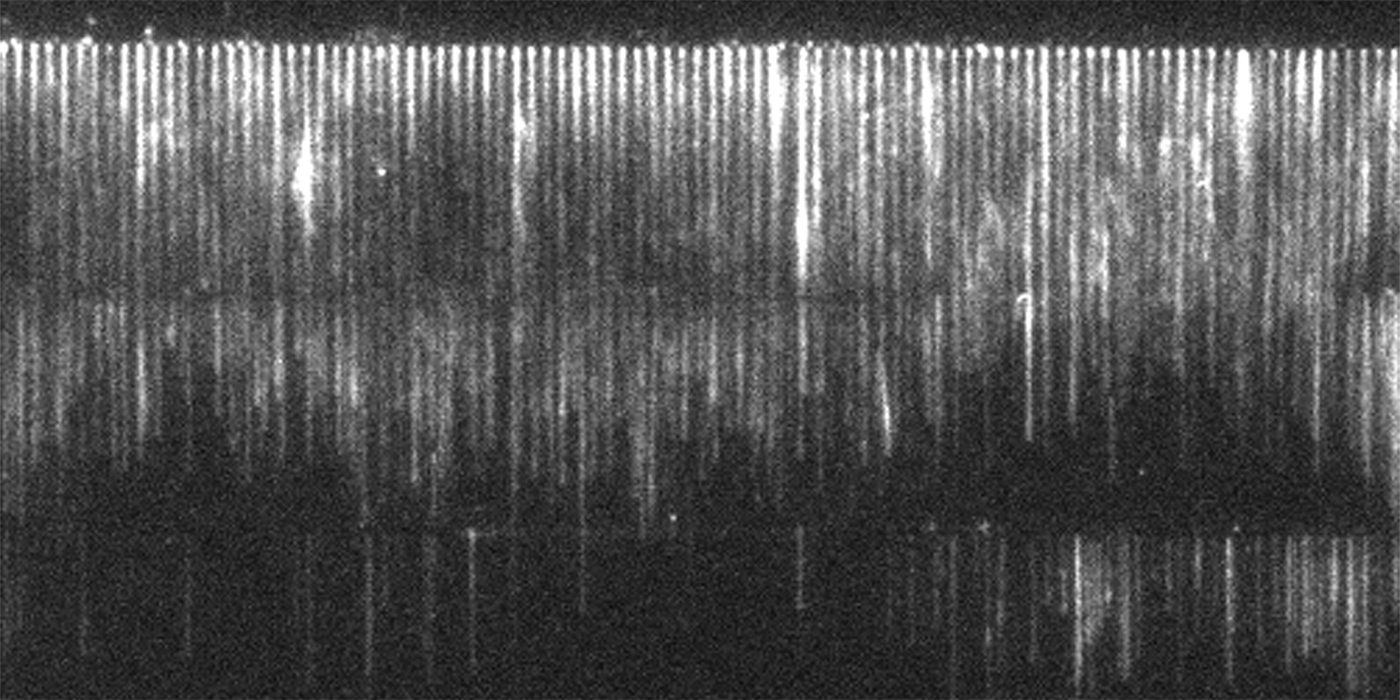
In physics, researchers often investigate the various forces that affect different components of a system. Since there wasn’t an optimal experimental construct allowing Courtemanche to analyze the relationship between force and polymerization rate in the actin-formin system, she invented her own.
Courtemanche’s technique—an elegant fusion of nanotechnology, microscopy, physics, and biology—creates stunning “actin curtains” that cascade serenely down the microscope slide. With hundreds of growing polymers arranged in sleek, lustrous rows, data analysis becomes conveniently tidy and efficient.
Courtemanche ultimately wants to understand how sequence differences between the 15 types of mammalian formins result in each of their idiosyncrasies. She will begin by characterizing the effects of varying the force applied to the actin curtains, and will eventually develop more complex systems involving actin bundles and other actin-binding proteins.
“At universities, you never know where you’re going to find inspiration,” says Courtemanche, who chanced upon her accidental idea at a departmental seminar during her post-doc at Yale. Eric Greene of Columbia University had invented DNA curtains in order to visualize fluorescently tagged DNA-binding proteins. “My boss and I, both at the same time, were like, we’ve got to do this with formins!” And so, in close collaboration with Greene, they did.
Courtemanche is thrilled to join the cytoskeletal community here on campus. “At the U of M, you’ve got the opportunity to ask a question, and then answer it in the best way you can, rather than building your research around where you're pigeon-holed,” she says. “I just love that.”
– Colleen Smith