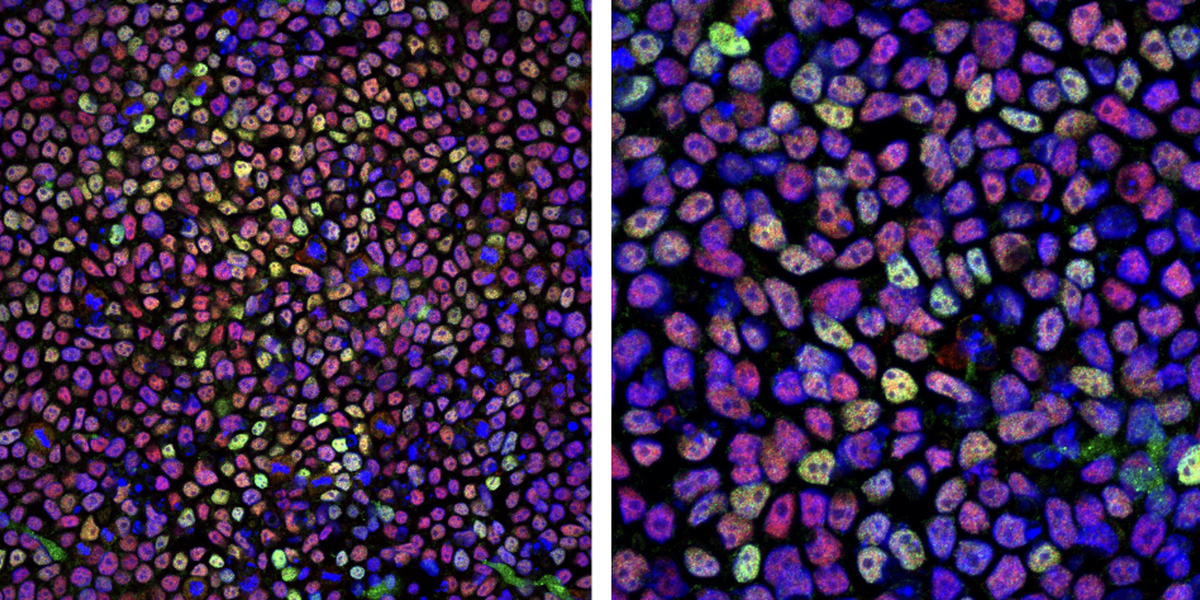
Images of the induced pluripotent stem (iPS) cells Santarriaga uses in her research.
Mental illness: nature or nurture? Science says both – the two are nearly indistinguishable in the complex ways they interact.
“What we know for sure [about individuals with schizophrenia] is that their neurons aren't functioning the way they should,” says Stephanie Santarriaga, a President’s Postdoctoral Fellow in the College of Biological Sciences’ Department of Genetics, Cell Biology and Development. “But what leads to this dysfunction is unclear. An individual may carry a genetic risk for various conditions, but that doesn’t mean they will develop a psychiatric disorder.”
How complex genetics and the cellular environment interact and lead to neuronal dysfunction is a topic of interest in Santarriaga’s lab. She’s also interested in how neuronal dysfunction is related to the different outcomes, or symptoms, that may be present in someone diagnosed with these disorders. “Schizophrenia has three different types of symptoms, and it’s not clear whether the pathways that lead to these different symptoms are the same, or completely different. It’s a very diverse condition,” says Santarriaga.
Thanks to advancements in stem cell research, Santarriaga can get a better picture of variables underpinning mental health and disease. She uses stem cells in her lab to replicate how brain cells of individuals with schizophrenia might function compared to those of healthy individuals. “My goal is that we can identify something that is perhaps dysfunctional that people didn't know about, that could be a new drug target.”
Manipulating the “original” cell
Before we had bones, muscles, blood and brains, we had stem cells. These cellular generalists have infinite potential (“they can make any cell in the body,” says Santarriaga) and each stem cell strain is as unique as the person they came from. Santarriaga uses stem cells on a daily basis and goes so far as to say each strain has a personality. “You’d never be able to tell by just looking at them,” laughs Santarriaga, “but over time we’ve started to recognize them just by how they behave or respond. I never expected that coming into this field.”
Advancements in stem cell technologies make it possible to reprogram mature human cells – like skin cells – back to their “original” stem-cell status. In Santarriaga’s case, her team collaborated with Massachusetts General Hospital to collect cells from both healthy individuals and individuals diagnosed with schizophrenia. After they’re reprogrammed by industry scientists, they’re sent as “blank slates” to Santarriaga.
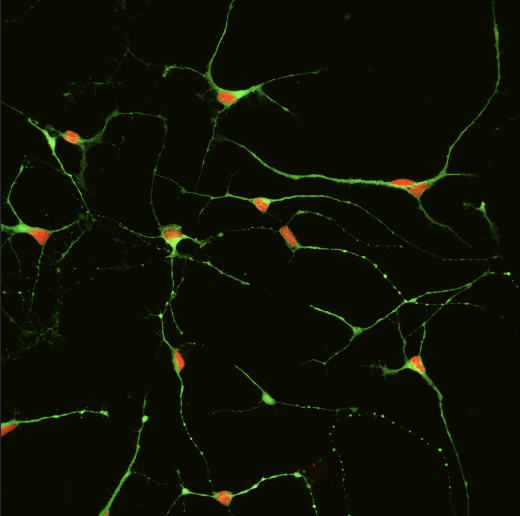
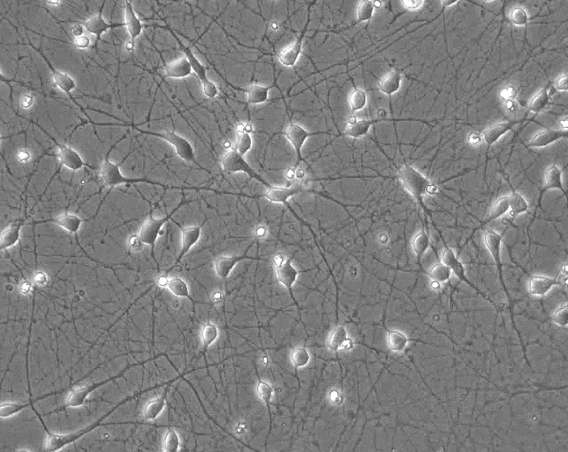
In her lab, Santarriaga gives her induced pluripotent stem (iPS) cells a new assignment: become a neuron. The small, two-dimensional neural networks she grows on petri dishes are alive; the neurons talk to each other, form structures, create byproducts, get healthy, get sick and die. In a way, she’s growing a tiny nervous system from the stem cells she acquires.
“Right now, I'm making two-dimensional layers with cortical neurons, but there's lots of different types [of neurons you can make from stem cells] to try to model different regions of the brain,” says Santarriaga. Cortical neurons can be found in the cerebral cortex, the brain’s outermost layer. “Another thing other people in the field find really interesting is that you can start with stem cells, and instead of making two-dimensional layers, you can make 3D structures, which are organoids, also known as mini-brains that have a more diverse cellular environment composed of glia and neurons.”
Santarriaga exposes her collection of neural networks to cellular stressors – like heat – and compares their cellular responses to otherwise healthy control groups. The possibilities of what part of the brain she could model or what kinds of induced responses she could measure seem almost infinite. But for now, she’s keeping things simple.
The brain’s version of street-sweeping
Alzheimer’s and other neurodegenerative diseases have been linked to a build-up of misfolded proteins in brain tissue. Santarriaga’s preliminary results show this is a characteristic that may be shared with schizophrenia. Cells grown from the stem cells of individuals with schizophrenia may also have trouble managing an excess of not-so-good proteins.
“If the [brain] cell is a city, then these protein clumps are like garbage that keeps piling up and isn't being removed,” says Santarriaga. “You can't traffic what you need to traffic and you begin to disrupt the cell as a whole.” She and her team use a specialized technique to visualize the protein aggregation under a microscope. “They show up as little dots that are basically clumps of proteins,” says Santarriaga. “My goal now is to identify what those clumping proteins actually are.”
Of course, the work is far from over. Once she finalizes her results, Santarriaga wants to increase the complexity of her experiment and describe how dysfunction plays out across other regions of the brain. Each region of the brain has a unique cocktail of specialized proteins, neurons, hormones and other cellular materials and environments that keep the brain functioning in a healthy balance. When these neurons misfire, the brain’s chemistry gets altered or a brain cell’s repair function is broken, lots can go wrong.
“A lot of psychiatric disorders are really under-treated,” says Santarriaga, who sees herself broadening her research to include bipolar and depression in the future. “I really hope this work creates a new avenue of research or assists in understanding what’s happening at the cellular level.” – Adara Taylor
Photo credit goes to Stephanie Santarriaga