Development of a genome-wide SNP map for Candida albicans
Single nucleotide markers are essential tools to study a variety of properties and processes in organisms, such as recombination, chromosomal dynamics, genome rearrangement, and the genetic relatedness between individuals. We developed a genome-wide SNP map for Candida albicans to study recombination as a genome-wide process. This map will also be a valuable tool to study population genetics, relationships of strains, and transmission genetics. Furthermore, heterozygosity can also be exploited to unravel processes contributing to the diversity and plasticity of the C. albicans genome. We defined a SNP marker as a small PCR product ranging in size between 100 and 400 bp and containing at least one single nucleotide polymorphism.
To design SNP markers for this map, we took advantage of the available genome sequence represented as Contigs-6 and the contig-19 assembly of sequencing contigs-6 at the Stanford C. albicans genome web site as well as several publicly available web pages. To view some of these site, click on the 'Additional Links' link at the top of the page.
We generated 119 potential marker sequences heterozygous in strain SC5314. Adding 31 markers from previous studies (1-4), a total of 150 SNP markers was obtained (see Table). The SNP marker sequences in the set of 150 ranged in size from 120 bp to 1,821 bp with an average of 322 bp. Once examined for heterozygosity in SC5314 by resequencing, we found that 19 markers, all from previous studies were homozygous. The remaining 131 SNP marker sequences were all heterozygous and revealed 561 single nucleotide polymorphisms and 9 insertions/deletions (indels) for a total of 570 polymorphisms.
The SNP map project is a collaboration of the laboratories of Dr. Georgiana May (Department of Ecology, Evolution and Behavior; joint appointment with the Department of Plant Biology) and P. T. Magee.
References
1. Cowen, L. E., C. Sirjusingh, R. C. Summerbell, S. Walmsley, S. Richardson, L. M. Kohn, and J. B. Anderson. 1999. Multilocus Genotypes and DNA Fingerprints Do Not Predict Variation in Azole Resistance among Clinical Isolates of Candida albicans. Antimicrob Agents Chemother 43:2930-2938.
2. Forche, A., G. Schönian, Y. Gräser, R. Vilgalys, and T. G. Mitchell. 1999. Genetic structure of typical and atypical populations of Candida albicans from Africa. Fungal Genet Biol 28:107-25.
3. Gräser, Y., M. Volovsek, J. Arrington, G. Schönian, W. Presber, T. G. Mitchell, and R. Vilgalys. 1996. Molecular markers reveal that population structure of the human pathogen Candida albicans exhibits both clonality and recombination. Proceedings of the National Academy of Sciences of the United States of America 93:12473-7.
4. Xu, J., T. G. Mitchell, and R. Vilgalys. 1999. PCR-restriction fragment length polymorphism (RFLP) analyses reveal both extensive clonality and local genetic differences in Candida albicans. Mol Ecol 8:59-73.
5. Forche, A., P.T. Magee, B.B. Magee, and G. May.2004. Development of a genome-wide SNP map for Candida albicans. Eukaryot. Cell 3: 705-714
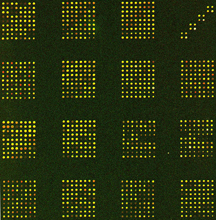
This figure shows an example of our whole-genome SNP array with 137 SNP markers. For each marker one SNP was chosen for the array. Two 30mer oligonucleotides for each SNP representing allele 1 and allele 2, respectively, are spotted next to each other. These oligonucleotides consist of a 15 nucleotide T-polylinker and a 15 nucleotide target sequence with the polymorphic base at the middle position. The 30mers are spotted onto an amino-activated glass slide and cross-linked via amino linker chemistry. Each oligo is spotted in 4 replicates yielding a total of 1096 spots. To account for differences in spotting intensities of spots, variation in hybridization of probes to the oligos on the slide, and to account for possible cross hybridization, we are using strain SC5314 as hybridization control. Probing the microarray with a single stranded, labeled PCR probe, we are able to identify all three possible genotypes (AA, BB, and AB) at each of these loci.