Research Interests
My primary research project is studying the dynamics of the SERCA-PLB complex using conventional and saturation transfer electron paramagnetic resonance (EPR). SERCA is an integral membrane protein located in the cellular sarcoplasmic reticulum (SR) that hydrolyzes ATP to pump calcium out of the SR, causing muscle relaxation. PLB is also an integral membrane protein, and inhibits SERCA activity upon binding. This inhibition can be reversed by phosphorylation of PLB, but the structural mechanism behind this relief of inhibition is not well understood. Elucidation of this interaction could lead to treatments for muscular defects, such as heart failure and muscular dystrophy. For example, overexpression of SERCA via AAV vectors has been shown to rescue heart failure in human clinical trials (Lipskaia et al 2010), as well as significantly increase muscle capacity in mice with muscular dystrophy (Goonasekera et al 2011).
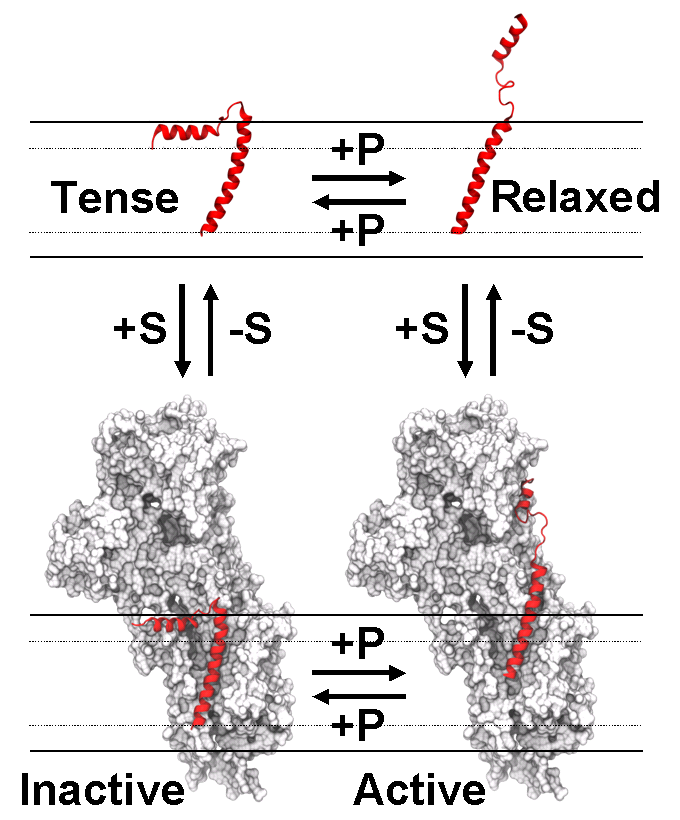
I study the SERCA-PLB interaction using electron paramagnetic resonance. We attach spin labels to specific locations on the protein with site-directed spin labeling (SDSL) and solid phase peptide synthesis (SPPS). The EPR experiments reveal static and dynamic properties of the spin label. Using a rigid spin label such as TOAC, we can extend these spin label properties to the protein itself. To study the SERCA-PLB complex, we co-reconstitute varying amounts of SERCA and TOAC-PLB (unphosphorylated and phosphorylated) into lipid vesicles, then acquire EPR spectra to determine the rotational mobility of PLB. These deviations in rotational mobility indicate important structural changes, including conformation and protein binding. Complimentary EPR experiments, such as dipolar broadening and dipolar electron-electron resonance (DEER), produce distance measurements between adjacent spin labels, so these techniques are also implemented to provide supporting information.
My novel contributions to this project include optimization of the conditions for PLB/SERCA co-reconstitution, calibration of ST EPR as a quantitative measure, and sequential variation of protein content to demonstrate incremental effects. For simplicity, these experiments currently use a synthesized monomeric form of PLB, however we intend to probe wild type PLB, which has been shown to exist in a dynamic equilibrium between monomeric and pentameric forms. Additionally, we will explore oligomeric interactions among SERCA molecules, because this is believed to change the binding dynamics with PLB. Since electrostatic interactions are critical in the SERCA-PLB interaction (MacLennan et al 1993), we will introduce charged lipids in the co-reconstitution vesicles to study the effect on binding affinity.