
Damaged DNA is bad news, and cancer and infertility are just a few of the health issues that can arise when DNA repair goes awry. Thankfully, sexually reproducing organisms have a range of tricks they play to ensure their genomes are passed on to their progeny intact, and researchers are learning from this process to lay the groundwork for medical breakthroughs.
Using advanced microscopy techniques on a tiny worm called C. elegans, Lexy von Diezmann — recipient of the Dale F. Frey Award for Breakthrough Scientists and new faculty member in the college's Department of Genetics, Cell Biology and Development — is investigating the basic principles behind DNA repair. Diezmann’s research fuels the fight against cancer and contributes to a growing understanding of how genetic and molecular signals are coordinated within cells.
Diezmann has a background in chemical physics and super-resolution imaging, and admits she never expected to become so immersed in the weird world of worms. It just so happens that the translucent tissue and simple anatomy of C. elegans makes it an ideal model organism to view under a microscope.
A screenshot displays an outline of tiny nematode organs glowing bright against black. Magnifying fluorescent labels makes the worm’s six chromosomes clearly visible within nuclei as they develop from stem cells into gametes (eggs or sperm). But what makes von Diezmann’s high-resolution imaging different from other techniques is that it can track single molecules as they explore chromosomes in three-dimensions. In other words, von Diezmann can observe individual proteins in real time while parental chromosomes replicate, align, are repaired or separate.
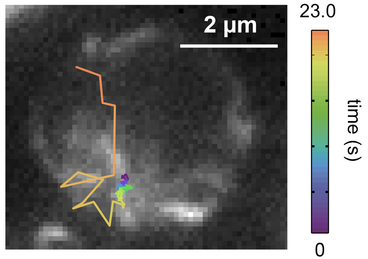
“You shoot very bright lasers into a really powerful objective lens, and then that same lens collects as much light as it can and shunts it to a camera,” says von Diezmann. “So you can see these individual glowing spots as they move around, kind of like fireflies, and watch their trails as they go through this dark environment.”
A set of von Diezmann’s recent time-lapse images give rare insight into the early stages of C. elegans’s sperm and egg cell production. Chromosomes appear as glowing squiggles in the tightly packed germ cells of the worm’s gonads. Over the span of a few seconds, a bright dot zig-zags through the glowing masses of DNA. It’s the voyage of ZHP-3, a protein known to be involved in mending DNA during genetic crossover events in which the genome undergoes a series of induced breaks and repairs to create a more diverse gene pool for progeny. The navigation of each ZHP-3 molecule looks a bit like a poorly executed connect-the-dots puzzle.
And for what it’s worth, crossover events are a bit chaotic. After corresponding chromosomes condense and pair up next to each other, DNA breaks are induced in a random distribution. Yet while the two chromosomes link together, the site where the two strands connect for recombination becomes fairly localized. Think of the point where lines in the letter “X” meet.
So how do crossover sites get selected? How do molecules communicate or interfere so the DNA recombines successfully?
Diezmann collected timelapse images of hundreds of individual ZHP-3 proteins to see if there were any noticeable themes in its movement. It was a lot more variable than they expected. “If you plot all the trajectories, you get this really wide variety of different dynamics that play out,” says von Diezmann. “This was one of the things that surprised us, just how heterogeneous it was.”
Some proteins were stuck, or “bound” as von Diezmann called it. Others diffused more freely along the chromosomes, or jumped on or off them. It doesn’t mean a failed experiment–von Diezmann thinks the variability in how the protein moved means there’s a richer mechanism at play than she first thought.
It’s the first step in a long list of variables to investigate. Applying new, more rapid imaging techniques and studying new proteins and genetic conditions are both on von Diezmann’s list for the new year. But studying weird worms has definitely grown on von Diezmann, who endearingly calls their collection of plated worm colonies “the ranch.” The stunning visuals they allow for keep von Diezmann hooked.
“You see the chemistry happening in real time, and it’s all spatially regulated and organized. You see these bustling blobs of molecules making decisions and trying to do their thing…It’s really powerful.” — Adara Taylor